The Lung-MAP (NCTID:03851445) Trial: Early Patient Tracking for Improved Accruals

The Lung-MAP trial also known as the Lung Cancer Master Protocol, is a precision medicine clinical trial designed for people with advanced non-small cell lung cancer (NSCLC) that has continued to grow after treatment. Enrollment in the Lung-MAP trial can provide critical access to targeted therapies for clinically appropriate patients.
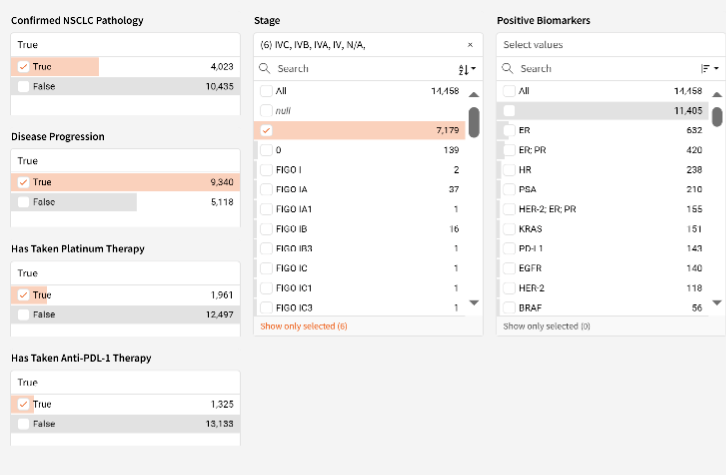
One barrier to patient enrollment is the trial’s complex screening criteria, which vary based on whether the patient is currently in stage 1 to 3, or stage 4. For patients to be eligible to enroll, they must have progressed on platinum-based therapy if they are currently metastatic or have progressed within one year of initiation of such therapy for patients who are not metastatic at diagnosis.
The need to monitor these criteria can be a barrier to research teams to identify patients in time. Utilizing AI-driven, automated mechanisms to identify individuals in the early stages of lung cancer who show signs of progression can help mitigate this problem. By monitoring these patients early on, through automated, real-time reports, patients can be identified, educated and pre-screened early.
In a recent implementation of OncoLens Patient ID, a digital pre-screening and tracking solution that combs through structured and unstructured EMR data and matching those to the trial’s inclusion/exclusion criteria, 8% of lung cancer patients were found to be potential candidates for the trial and placed in a tracking queue. 2% ultimately accrued while simultaneously reducing the time to find patients from 5 hours per week to less than 30 minutes.
Learn more about OncoLens Patient ID solutions.
A sample of OncoLens’s proprietary data extractors from structured and unstructured EMR data including pdf documents